Abedz N, Pawliczak R. Efficacy and safety of topical calcineurin inhibitors for the treatment of atopic dermatitis: meta-analysis of randomized clinical trials. Postepy Dermatol Alergol 2019; 36(6): 752-759.
Ashcroft DM, Chen LC, Garside R, Stein K, Williams HC. Topical pimecrolimus for eczema. Cochrane Database Syst Rev 2007; (4): CD005500.
Chi CC, Wang SH, Wojnarowska F, Kirtschig G, Davies E, Bennett C. Safety of topical corticosteroids in pregnancy. Cochrane Database Syst Rev 2015; (10): CD007346.
Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev 2015; (7): CD009864.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71(1): 116-132.
Gonzalez-Lopez G, Ceballos-Rodriguez RM, Gonzalez-Lopez JJ et al. Efficacy and safety of wet wrap therapy for patients with atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol 2017; 177(3): 688-695.
Green C, Colquitt JL, Kirby J, Davidson P, Payne E. Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation. Health Technol Assess 2004; 8(47): 1-120.
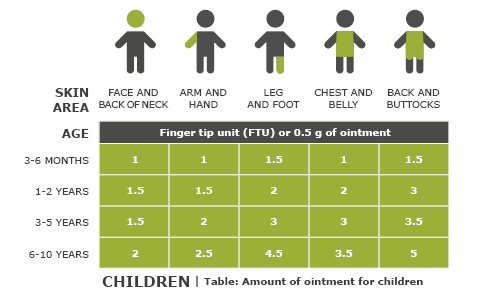
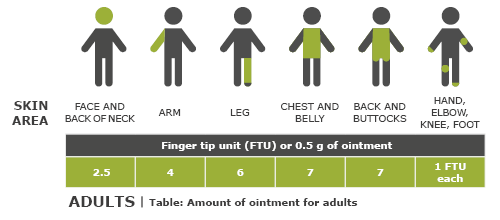
Phillips R, Williams H, Ravenscroft J. Management of atopic eczema in children. Prescriber 2016; 27(1): 33-37.
Ridd MJ, Roberts A, Grindlay D et al. Which emollients are effective and acceptable for eczema in children? BMJ 2019; 367: l5882.
Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2011; 164(2): 415-428.
Sigurgeirsson B, Boznanski A, Todd G et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics 2015; 135(4): 597-606.
IQWiG health information is written with the aim of helping people understand the advantages and disadvantages of the main treatment options and health care services.
Because IQWiG is a German institute, some of the information provided here is specific to the German health care system. The suitability of any of the described options in an individual case can be determined by talking to a doctor. informedhealth.org can provide support for talks with doctors and other medical professionals, but cannot replace them. We do not offer individual consultations.
Our information is based on the results of good-quality studies. It is written by a team of health care professionals, scientists and editors, and reviewed by external experts. You can find a detailed description of how our health information is produced and updated in our methods.